 |
 |
| Physiology? | Figures & Illustrations | Test Questions | Daily Quiz | Calculators | Physiology Tutor | Glossary |
 |
 |
 |
 |
 |
 |

Resting Membrane Potential -
Maintenance of the Membrane Potential
Maintenance of the Membrane Potential
Now that we have a good understanding of how the membrane potential comes about (see here) and the factors that govern the value of the membrane potential (see here), we now wish to better understand how the membrane potential is maintained in cells.
We have already seen that in order to establish a membrane potential, all that is needed is asymmetric ion distribution (i.e., concentration gradient) and selective ion channels. We have also seen that in most cells the resting Vm (Vrest) is determined by K+, Na+, and Cl−. Since in most cells Vm is not at the equilibrium potential for either K+, Na+, or Cl−, at the resting membrane potential, each ion is moving down its own electrochemical gradient according to the driving force acting on the ion. As we saw before, the driving forces can be described by the following:
| DFK = Vm − VK = −68 − (−97) = +29 mV | Eq. 1 |
| DFNa = Vm − VNa = −68 − (+61) = −129 mV | Eq. 2 |
| DFCl = Vm − VCl = −68 − (−64) = −4 mV | Eq. 3 |
For a typical Vm of −68 mV, you should now be able to determine the direction of ion flow for K+, Na+, and Cl− (see the previous section and the electrochemical driving force calculator).
Thus, it is clear that under resting physiological conditions, there is constant efflux of K+ from the cell, influx of Na+ into the cell, and efflux of Cl− from the cell (Fig. 1). Therefore, if the ionic concentration gradients are not maintained, in the long run, because of the constant ionic fluxes across the plasma membrane, the concentration gradients will be dissipated. Cells avoid this situation by having a primary active transporter (i.e., pump) which pumps Na+ out of the cell and K+ into the cell to counteract the constant movements of these two ions down their electrochemical gradients (Fig. 1). This protein is the Na+/K+ ATPase (commonly also referred to as the Na+ pump) which couples the hydrolysis of one ATP molecule to moving 3 Na+ ions out of the cell and 2 K+ ions into the cell.

Figure 1. Steady-state fluxes of K+ , Na+, and Cl− across the plasma membrane under resting physiological conditions.
Under resting physiological conditions, because of the driving forces acting on K+, Na+, and Cl− (see figure), there is constant efflux of K+ out of the cell, influx of Na+ into the cell, and efflux of Cl− out of the cell. Note that the sizes of the arrows shown do not accurately represent the magnitudes of the fluxes. See the section on Membrane Ionic Current Equations and Chord Conductance Equation for details.
It is noteworthy that in many cells, the contribution of Cl− to the membrane potential is small and VCl usually (but not always) follows Vm. This means that Cl− distributes across the plasma membrane to adjust the intracellular concentration to establish a VCl that is close to Vm. While in these cases, Cl− may not contribute greatly to the resting membrane potential, the existing Cl− permeability does guard against abrupt and large changes in the membrane potential. As we will see in future lectures, this is extremely important in excitable cells such as neurons.
If the activity of the Na+/K+ ATPase is inhibited in a cell (for example with vanadate or ouabain; pronounced "wah-bane"), initially the membrane potential is not altered (or is altered very slightly) (Fig 2). During every ATP hydrolysis and transport cycle, the Na+/K+ ATPase transports 3 Na+ ions out of the cell and 2 K+ ions into the cell leading to an electrogenic transport process. Thus, during every transport cycle of the Na+ pump, one net positive charge is translocated across the plasma membrane (inside to outside) and, therefore, pump activity contributes very slightly to the value of the negative membrane potential. The magnitude of the contribution varies from cell to cell and depends largely on other ionic pathways in the plasma membrane. Thus, inhibition of the Na+/K+ ATPase usually leads to a minor (less than 5 mV) depolarization of the plasma membrane. It is important to notice is that inhibition of the Na+/K+ ATPase does not immediately abolish the membrane potential.
However, if the pump is not allowed to function for a long period of time (> tens of seconds), the membrane potential begins to become less negative until it ultimately completely disappears (i.e., Vm = 0) (Fig. 2). At this point, the cell is dead! This is because constant Na+ influx into the cell and K+ efflux out of the cell serve to abolish the transmembrane Na+ and K+ concentration gradients. In the absence of concentration gradients, the Nernst potential for each of these ions would be close to zero leading to a membrane potential of approximately zero. Thus, in order to maintain the membrane potential, cells have to expend energy to maintain the proper intracellular Na+ and K+ concentrations. Therefore, although the Na+/K+ ATPase is not responsible for the generation of the membrane potential, it is responsible for its maintenance by maintaining the normal intracellular K+ and Na+ concentrations.
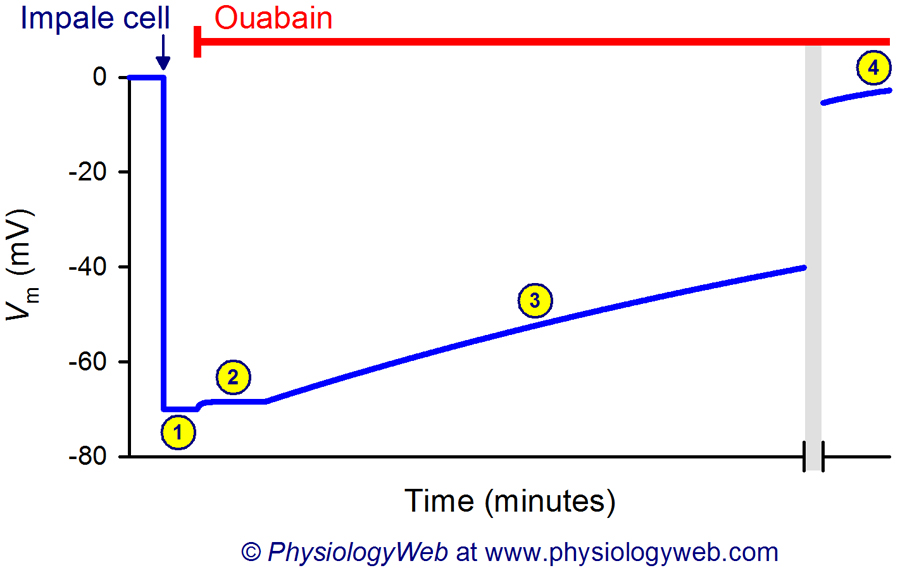
Figure 2. Inhibition of the Na+/K+ ATPase leads to the ultimate loss of the membrane potential.
Inhibition of the Na+/K+ ATPase by ouabain leads to a rapid but very small depolarization of the membrane (2). The magnitude of this depolarization is generally small (less 5 mV) and depends on the presence of other ionic pathways in the membrane (i.e., input resistance of the membrane). In some cells, no initial depolarization is observed when the sodium pump is inhibited (because the input resistance is low due to the high permeability of other ionic pathways). However, prolonged inhibition of the Na+/K+ ATPase leads to a gradual dissipation of transmembrane Na+ and K+ concentration gradients and, thus, a gradual decline of the membrane potential until its value reaches zero (3 and 4). The gray bar represents a long break in time, indicating that it takes a long time (tens of seconds or minutes) for the membrane potential to completely dissipate to the zero level (4).
At this point, it may be wondered what maintains the extracellular concentrations of K+ and Na+. Two organ-systems are responsible for maintaining the low extracellular K+ concentration and the high extracellular Na+ concentration. The kidneys (urinary system) working in conjunction with the endocrine system are responsible for extracellular K+ and Na+ homeostasis (see lecture notes on Renal Physiology).
Posted: Saturday, February 15, 2014
Last updated: Thursday, November 23, 2017
Last updated: Thursday, November 23, 2017