 |
 |
| Physiology? | Figures & Illustrations | Test Questions | Daily Quiz | Calculators | Physiology Tutor | Glossary |
 |
 |
 |
 |
 |
 |

Neuronal Action Potential -
Important Features of the Neuronal Action Potential
Important Features of the Neuronal Action Potential
When the stimulus given to a resting neuron is supra-threshold, it results in an action potential. As long as the depolarization caused by the stimulus is above threshold, the size of the resulting action potential will be the same (Fig. 1). In other words, once the membrane potential surpasses threshold, it will uncontrollably go to the peak of the action potential at around +50 mV (near the Na+ equilibrium potential, VNa). So long as threshold is surpassed, additional increases in stimulus strength do not lead to increases in the magnitude of the voltage deflection of the action potential. This is referred to as the all-or-nothing law, and refers to the fact that there is no "in-between" action potential (Fig. 1). The neuron either does not respond (in the case of sub-threshold stimuli), or it will generate a full-fledged, all-or-nothing action potential (in the case of supra-threshold stimuli). See the section on Frequency Coding in the Nervous System in this lecture for how the nervous system encodes varying strengths of supra-threshold stimuli.

Figure 1. Action potentials are all-or-nothing.
So long as threshold is reached, further increases in the amplitude of the stimulus (top, red trace) do not cause increases in the size of the voltage deflection of the action potential (bottom, blue trace). The two traces are on the same time scale. The dashed line represents the threshold voltage (Vthreshold) of approximately −50 mV. Here, a threshold stimulus refers to that which is just strong enough to bring a resting neuron to threshold.

Figure 2. Important features of the neuronal action potential.
Different phases of the neuronal action potential are emphasized in this figure. 0, resting state; 1, depolarization to threshold and beyond; 2, overshoot; 3, peak of the action potential; 4, repolarization; and 5, hyperpolarization. See text for details.
Now let us summarize the events that take place during an action potential. The action potential can be divided into five phases (Fig. 2): (1) depolarization to and beyond threshold, (2) overshoot, (3) peak, (4) repolarization, and (5) hyperpolarizing afterpotential (or simply hyperpolarization) (Fig. 2). Letís consider each of these events in detail.
As mentioned above, if the initial depolarization does not reach the threshold voltage, no action potential will result. However, if depolarization is large enough such that the membrane potential reaches −50 mV, a complete all-or-nothing action potential will result. As will be discussed below, unique voltage-gated ion channels, namely voltage-gated Na+ and K+ channels, respond to membrane depolarization to or beyond the threshold voltage. Remarkably, all of the features of the action potential can be explained by understanding the molecular properties of these voltage-gated ion channels, and the electrochemical gradients of Na+ and K+ across the neuronal plasma membrane.

Figure 3. The Hodgkin cycle.
The Hodgkin cycle represents a positive feedback loop in which an initial membrane depolarization leads to uncontrolled deflection of the membrane potential to near VNa. The initial depolarization must reach or surpass threshold in order to activate voltage-gated Na+ channels. Opening of Na+ channels allows Na+ inflow which, in turn, further depolarizes the membrane. Additional depolarization activates additional Na+ channels. This cycle leads to a very rapid rise in Na+ conductance (gNa), which moves the membrane potential close to VNa. Condunctance can be thought of as the electrical counterpart of permeability (p). Both conductance and permeability are proportional to the total number of open channels in the membrane.
The uncontrolled depolarization that takes place (also referred to as the spike phase of the action potential; 1 in Fig. 2) is strictly a function of voltage-gated Na+ channels in neurons. At rest (−70 mV), the voltage-gated Na+ channels are closed, but begin to open at membrane potentials ranging from −40 to −50 mV (threshold voltage, Vth). Opening of Na+ channels leads to the entry of a large amount of Na+ ions into the cell. Remember that a very large driving force (~100 mV) acts on Na+ ions favoring their movement into the cell through now open Na+ channels. This Na+ influx is brought about both by the Na+ concentration gradient, and the inside negative membrane potential. Entry of Na+ into the cell brings about further depolarization. Membrane depolarization further activates additional Na+ channels which, in turn, leads to the entry of more Na+ into the cell. Therefore, a positive feedback loop is established, which leads to increasing entry of Na+ into the cell. This positive feedback loop is called the Hodgkin cycle (Fig. 3), so named because of the investigator who pioneered most of what we know today about electrical activity in neurons. Thus, the Hodgkin cycle is responsible for the spike phase of the action potential.
The continued entry of Na+ into the cell leads to rapid depolarization of the cell (< 1 ms). Because rapid opening of Na+ channels leads to a rapid rise in membrane permeability to Na+, the membrane potential reverses its sign (goes from negative to positive) and approaches the equilibrium potential for Na+ (about +50 mV). Remember from the previous lecture on the resting membrane potential that the movement of an ion down its electrochemical gradient tends to move the membrane potential towards the equilibrium potential for that ion. In this case, Na+ entry into the cell through voltage-gated Na+ channels takes the membrane potentials close to the Na+ equilibrium potential (VNa). Reversal of the sign where the membrane potential becomes positive is referred to as overshoot (2 in Fig. 2). Whereas at rest, the relative permeabilities of K+ (pK) and Na+ (pNa) are 1 : 0.05 (pK : pNa), at the peak of the action potential, the pK : pNa ratio is about 1 : 12. However, keep in mind that at the peak of the action potential the absolute value of pK is also larger than its value at rest (see Fig. 5 below). At the peak of the action potential pNa is about 600 times greater than its resting value, whereas pK is about 3 times its resting value. If you use the Goldman-Hodgkin-Katz (GHK) equation and the permeability values at the peak of the action potential (pK : pNa ratio of 1 : 12), you can convince yourself that Vm approaches VNa. While the maximum pNa is observed at the peak of the action potential, the maximum value of pK is observed shortly after the peak of the action potential (see Fig. 5 below).
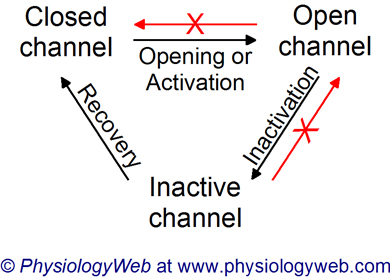
Figure 4. Na+ channel activation, inactivation, and recovery from inactivation.
Neuronal voltage-gated Na+ channels can exist in one of three different conformations: closed state, open state, and inactive state. At the resting membrane potential, Na+ channels are closed (non-conducting; pNa = 0). Membrane depolarization to the threshold voltage leads to the opening (activation) of voltage-gated Na+ channels (channels conduct ions; pNa > 0). The channels then spontaneously enter an inactive state (non-conducting state; pNa = 0). Recovery from inactivation is a time- and voltage-dependent process and takes about 3-5 ms. Channels in the open state do not directly return to the closed state without entering the inactive state first. Inactive channels cannot enter the open state without returning to the closed state first. Even though the channel does not conduct Na+ ions in either the closed or inactive state (pNa = 0), the two states represent separate and distinct conformations of the channel.
At the peak of the action potential, the membrane potential is close to VNa, but it never reaches VNa (3 in Fig. 2). There are two reasons for this. First, the voltage-gated Na+ channels begin to inactivate spontaneously very rapidly after opening. Channel inactivation "plugs" the pore of the channel so that Na+ ions can no longer pass through the channel permeation pathway. A cytosolic region of the Na+ channel actually blocks the Na+ permeation pathway of the channel. This has been referred to as the ball-and-chain model of inactivation (Fig. 4). Ball refers to a globular cytoplasmic portion of channel protein that is tethered to the rest of the protein by a linker (or chain) part. A schematic representation of this process is shown in Fig. 4.
The second reason for the fact that the peak of the action potential does not reach VNa is that neurons also have voltage-gated K+ channels that become activated by membrane depolarization (also at around the threshold voltage of −40 to −50 mV). Activation of the voltage-gated K+ channels, however, is much slower than that of voltage-gated Na+ channels (Fig. 5). For this reason, these K+ channels are referred to as delayed rectifiers. Therefore, at the peak of the action potential, pK is greater than its value when the neuron is at rest, and movement of K+ out of the cell opposes the depolarization caused by the movement of Na+ into the cell (Fig. 5)
Once the peak of the action potential is reached, Na+ channels inactivate, and as a result pNa falls rapidly with time, and approaches its value at rest (Fig. 5). At this time, however, because of the delayed response of the voltage-gated K+ channels to membrane depolarization, pK is still becoming larger. Now the balance of ion flow across the membrane is in favor of K+ moving out of the cell. Movement of K+ out of the cell brings about rapid repolarization of the membrane back to the resting value (4 in Fig. 2). However, pK remains elevated for some time even after Vm has reached the resting value (Fig. 5). Therefore, continued movement of K+ out of the cell causes a membrane hyperpolarization (i.e., more negative than Vrest). This phase is commonly referred to as the hyperpolarizing afterpotential or simply hyperpolarization (5 in Fig. 2). This is also sometimes referred to as undershoot. This occurs because during this time pNa is at its resting value, but pK is higher than its resting value. Therefore, K+ movement out of the cell will tend to move the Vm closer to VK. Finally, pK returns to its value at rest, and at this time the membrane potential also returns to baseline at its resting value of about −70 mV. It is important to note that K+ channels do not inactivate. They close simply because the membrane potential becomes more negative than the threshold potential (the potential at which Na+ and K+ channels become activated). Thus, the repolarization and hyperpolarization that is caused by movement of K+ out of the cell through the voltage-gated K+ channels, also causes the closing of the same voltage-gated K+ channels.

Figure 5. Relative changes in pNa and pK during the action potential.
Relative changes in Na+ permeability (pNa) and K+ permeability (pK) are shown during the action potential. The membrane potential (Vm) is shown on the left vertical axis, and the relative permeabilities are shown on the right vertical axis. The relative permeabilites for Na+ and K+ are not drawn to scale. At the peak of the action potential, pNa is ~12 times larger than pK. See text for details.
Posted: Thursday, July 5, 2012
Last updated: Sunday, February 16, 2014
Last updated: Sunday, February 16, 2014